- ホーム
- SERVICE
- Business Registration
- Confirm required permissions
Business Registration
Confirm required permissions
Permission required for each medical device class, such as manufacturing / marketing license / manufacturing license / foreign manufacturer certification / highly managed medical device sales license
In order to sell a new medical device, you must apply for manufacturing and sales, manufacturing, sales, repair, and various business licenses

Work License Notification Flow
Confirmation of necessary licenses (Manufacturing / marketing license / manufacturing license / foreign manufacturer accreditation / sales permit for highly managed medical equipment, etc.)
⬇︎
Confirmation of basic requirements for products and confirmation of manufacturing sites
⬇︎
GQP / GVP / QMS systems and procedures
⬇︎
Application for permission and inspection
⬇︎
Get permission / authentication

Classification (I)
Permission required by the manufacturer / distributor
... Type 3 medical device manufacturing and sales license
Case of importing medical equipment
... Foreign manufacturer registration
Classification (Ⅱ)
Permission required by the manufacturer / distributor
... Type 2 medical device manufacturing and sales license
Permission required for sales offices and stores
... Managed Medical Equipment Sales Notification
Case of importing medical equipment
... Foreign manufacturer registration
Classification (Ⅲ)
Permission required by the manufacturer / distributor
... Type 1 medical device manufacturing and sales license
Permission required for sales offices and stores
... Sales license for highly managed medical equipment
Case of importing medical equipment
... Foreign manufacturer registration
Improvement of system
The government shall establish standards (systems) for systems related to the production control or quality control of medical devices or in-vitro diagnostic pharmaceuticals, standards for manufacturing control and quality control (QMS), and standards for post-marketing safety management of medical devices (GVP) has been promulgated as a ministerial ordinance.
Licensed medical device manufacturers must properly manage the quality and safety of medical devices in accordance with these ministerial ordinances.
The establishment of such a system is a requirement for “obtaining permission” and “maintaining permission”.
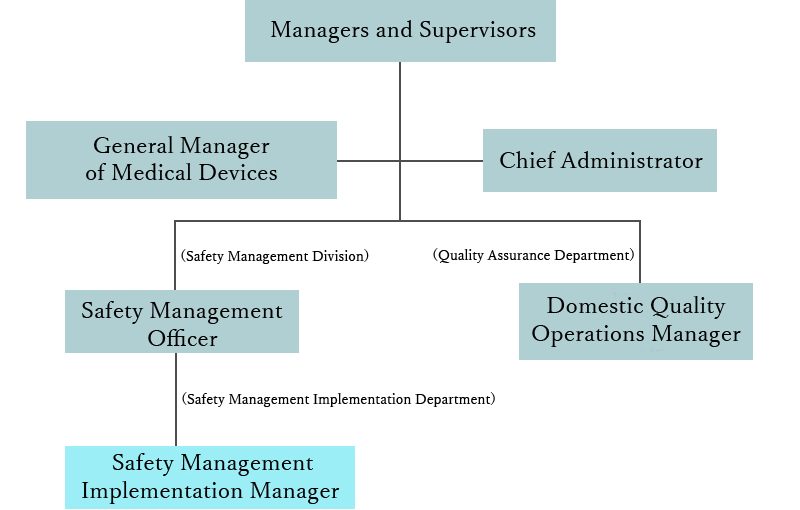
In the case of obtaining a manufacturing and marketing license, in addition to the management supervisor (manager) and the manager, the so-called three roles (general manager of medical equipment, manager of domestic quality business operation, safety management of medical equipment) must be placed.
When applying for a manufacturing registration, a responsible engineer must be assigned.

The Regulatory Affairs Organization provides detailed support for the creation and recording of documents and procedures required to obtain a business license.
For any requests or inquiries regarding the above consulting services, please do not hesitate to contact us via telephone or the inquiry page.
To request / inquiry form