- ホーム
- SERVICE
- Business Registration
- List of business license notification services
Business Registration
List of business license notification services
Detailed support for creation and recording of documents and procedures required for various business licenses such as manufacturing and sales / manufacturing / sales / repair business

In order to sell a new medical device, you must apply for manufacturing and sales, manufacturing, sales, repair, and various business licenses.
In order to continue to sell products, it is necessary to respond to QMS surveys and renewal of the certificate of conformity.
List of services
・ Support for creating new, relocating and renewing applications for manufacturing and sales
・ Support for creating business license applications for manufacturing, repair, and sales businesses
・ Confirmation before entering the work permit
・ Confirmation of QMS system, support for creating procedure manuals
・ In addition, consulting on application and notification of work license
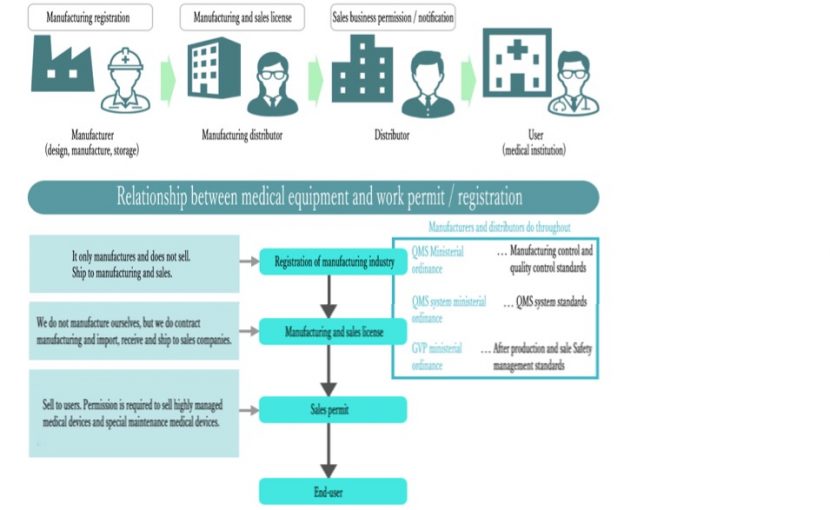
Work License Notification Flow
Confirmation of necessary licenses (Manufacturing / marketing license / manufacturing license / foreign manufacturer accreditation / sales permit for highly managed medical equipment, etc.)
⬇︎
Confirmation of basic requirements for products and confirmation of manufacturing sites
⬇︎
GQP / GVP / QMS systems and procedures
⬇︎
Application for permission and inspection
⬇︎
Get permission / authentication

The Regulatory Affairs Organization provides detailed support for the creation and recording of documents and procedures required to obtain a business license.
For any requests or inquiries regarding the above consulting services, please do not hesitate to contact us via telephone or the inquiry page.
To request / inquiry form